| Tweet |

上図がself exlanatoryと思うが、海の波ではなく、海水と淡水の混合環境を利用した安価な電力創出である。本文とそのURLは下記。
http://www.newscientist.com/article/mg20126972.000-salt-solution-cheap-power-from-the-rivers-mouth.html
Salt solution: Cheap power from the river's mouth
STAND on the banks of the Rhine where it flows into the North Sea, near the port of Rotterdam in the Netherlands, and you'll witness a vast, untapped source of energy swirling in the estuary. According to Dutch engineer Joost Veerman, it's possible to tap this energy without damaging the environment or disrupting the river's busy shipping. For rather than constructing a huge barrage or dotting the river bed with turbines, Veerman and his colleagues at Wetsus, the Dutch Centre for Sustainable Water Technology in Leeuwarden, believe they can tap energy locked up in the North Sea's saltwater by channelling it, along with fresh water from the Rhine, into a novel kind of battery. With a large enough array of these batteries, he says, the estuary could easily provide over a gigawatt of electricity by a process they've called Blue Energy - enough to supply about 650,000 homes.
"Salinity power" exploits the chemical differences between salt and fresh water, and this project only hints at the technology's potential: from the mouth of the Ganges to the Mississippi delta, almost every large estuary could produce a constant flow of green electricity, day and night, rain or shine, without damaging sensitive ecosystems or threatening fisheries (see map). One estimate has it that salinity power could eventually become a serious power player, supplying as much as 7 per cent of today's global energy needs.
In an attempt to prove that this isn't just a pipe dream, Veerman's team has done lab tests on a prototype salinity power generator, and are now planning to scale it up. Yet a group of Norwegian engineers have gone one stage further, with their own twist on salinity power.
In the next few months, engineers at Norwegian power company Statkraft plan to throw the switch on the world's first salinity power station. Though their prototype is small, its impact could be huge. So what are these rival technologies, how do they stack up, and what are the obstacles to making electricity wherever rivers meet the sea?
Salinity power emerged from a rather different use for sea water. In the late 1950s, Sidney Loeb and Srinivasa Sourirajan, then working at the University of California, Los Angeles, came up with a new trick to extract drinking water from the sea. Their idea was based on osmosis, a natural process in which water passes spontaneously from a dilute to a concentrated solution through a semipermeable membrane. The pair realised that by using a synthetic membrane and high pressure pumps, they could run osmosis in reverse and literally squeeze fresh water from sea water. This approach is now used in desalination plants worldwide.
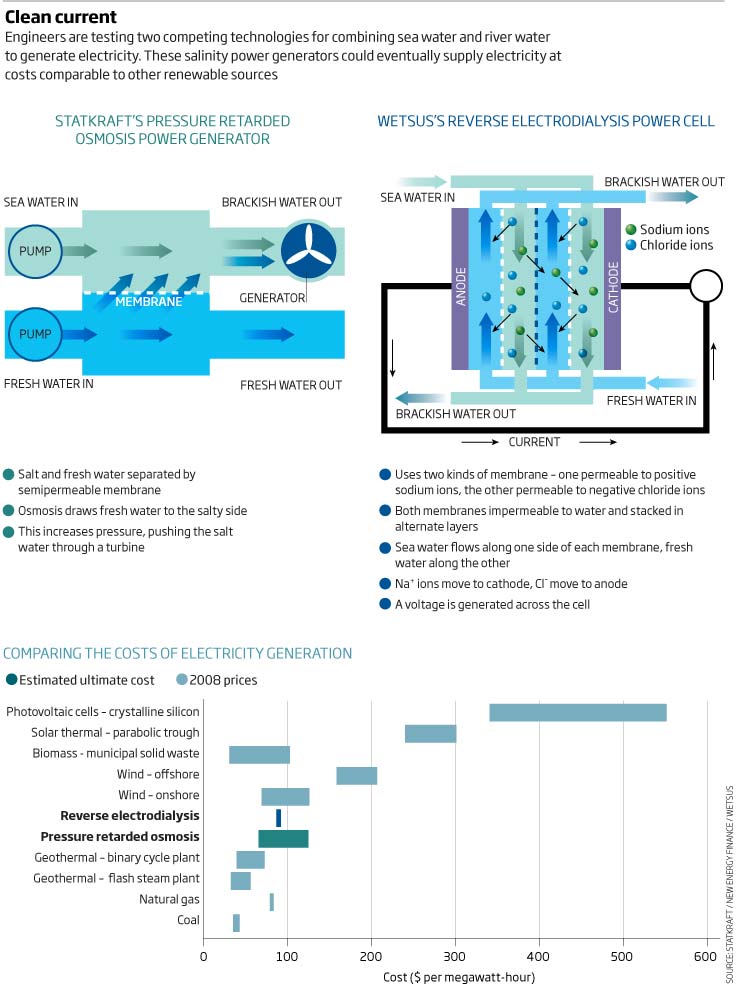
About 15 years later, Loeb had another brain wave. He realised that their design could be exploited to generate power. Working at Ben-Gurion University of the Negev, in Beer Sheva, Israel, he envisaged a tank with two chambers separated by a semipermeable membrane. With saltwater on one side and fresh on the other, osmosis would draw fresh water into the salty side, raising its pressure. This pressurised saltwater could then be piped through a turbine to generate electricity (see diagram). Loeb named this process pressure retarded osmosis (PRO) and patented it in 1973.
His plan was to harvest power where rivers meet the ocean, close to the point where fresh water meets salt. Fresh water would be piped to a generating plant from upstream and saltwater from downstream. Inside the plant, the fresh and saltwater would be channelled along either side of a membrane. Osmosis would then provide sufficient water pressure on the salty side of the membrane - up to 12 atmospheres, Loeb reckoned - to make electricity generation profitable.
The key lay in finding the right membrane. It would have to be permeable to water but not salt, and very thin yet extremely durable. This proved too tall an order and Loeb retired in 1986, his dream unrealised.
The concept was revived in 1997, when Thor Thorsen and Torleif Holt, working in Trondheim at the Norwegian research organisation SINTEF, became convinced that membrane technology was finally advanced enough to make Loeb's idea feasible. With their enthusiasm, and detailed calculations, they convinced Statkraft that salinity power could pay off in Norway. Using a design much like Loeb's original, they now believe they are close to their goal.
Membrane development remains the biggest headache, says Stein Erik Skilhagen, manager of the PRO project at Statkraft. Unfortunately, membranes used in desalination plants are too thick, he says, and cannot draw enough water through. So Statkraft's engineers have been working with membrane developers to improve designs. While their first membranes generated about 100 milliwatts per square metre, the latest version generates over 3 watts per square metre, close to their target of 5 watts.
Skilhagen reckons these membranes are now efficient enough to be worth testing beyond the lab, and in the next few months the company plans to turn on the world's first prototype PRO plant at the Södra Cell paper pulp factory in Tofte, alongside a fjord 60 kilometres from Oslo.
Read full article
Continue reading page |1 |2 |3
|
|
|
|
投稿コメント全ログ コメント即時配信 スレ建て依頼 削除コメント確認方法
|
|
 題名には必ず「阿修羅さんへ」と記述してください。
題名には必ず「阿修羅さんへ」と記述してください。
掲示板,MLを含むこのサイトすべての
一切の引用、転載、リンクを許可いたします。確認メールは不要です。
引用元リンクを表示してください。
|
|
|
|
|
|
|
|